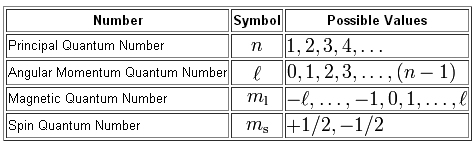
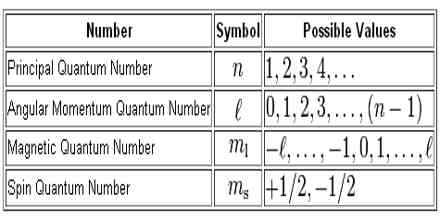
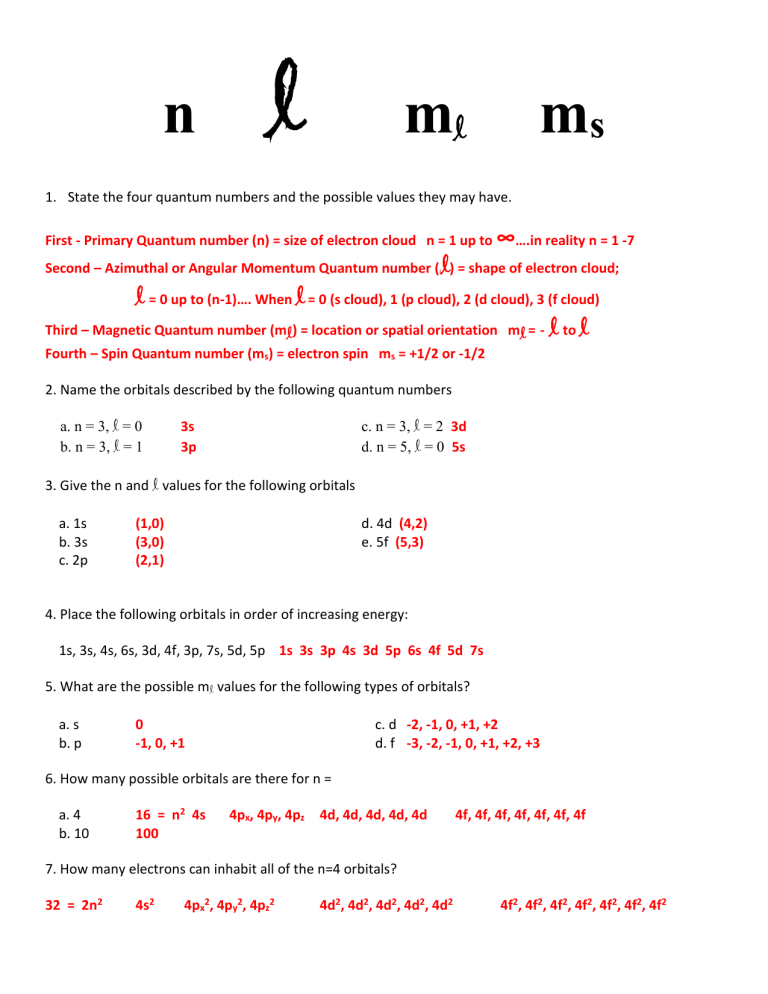
List the Four Quantum Numbers and Describe Their Significance
The principal quantum number - n The azimuthal or angular quantum number -. The orbital angular momentum quantum number l determines the shape of an orbital and therefore.
Quantum Numbers Introduction To Chemistry
As n increases the electrons energy and its average distance from the nucleus increase.
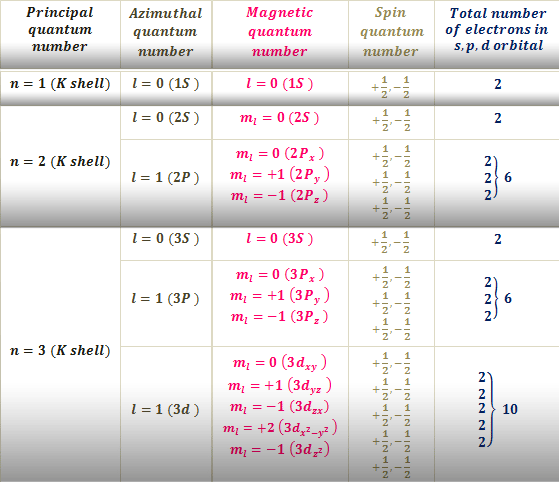
. The values of l allowed are. It can have values such as 1234etc. N 1 number of electrons.
List the four quantum numbers and describe their significance. SECONDARY QUANTUM NUMBER l - Represents the energy sublevel or type of orbital occupied by the electron. Later a fourth quantum number was added the measurement of spin.
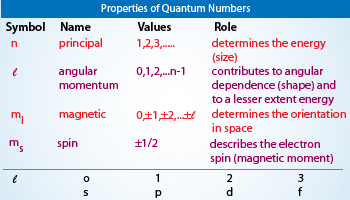
Sec 4-2 The Quantum Model of the Atom Heisenburg Uncertainty Principle Quantum theory Orbital Quantum number Principal quantum number Angular momentum quantum number Magnetic quantum number Spin quantum number Sublevel Degenerate orbitals Sec 4-3 Electron Configurations Electron configuration Aufbau principle Pauli exclusion principle. The allowed values and general meaning of each of the four quantum number of an electron numbers of an electron in an atom are as follows. The principal quantum number n designates the principal electron shell.
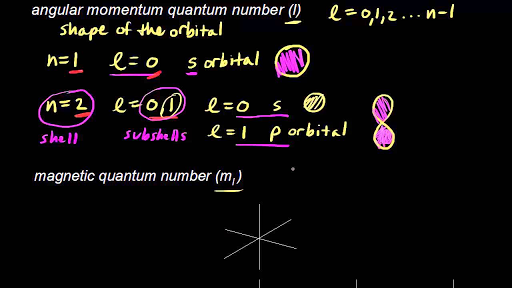
Describes the orbital of the subshell. The quantum number of orbital angular momentum also known as the azimuthal quantum number is indicated by the letter l. For example the 3d subshell is in the n 3 shell the 2s.
Describes the energy level. 1Principal quantum number nThis quantum number is the one on which the energy of an electron in an atom principally depends. The names of the quantum numbers are.
The quantum number that indicates the energy and orbital of an electron in an atom The angular momentum quantum number l. Principal Quantum Number n- indicates the main energy level occupied by the electron. Originally there were three quantum numbers the ones needed for the calculation of the key equation of quantum physics Schrodingers Equation.
ℓ - azimuthal or angular momentum quantum number. Quantum numbers describe specific properties of an electron. The principal quantum number n.
There can be two electrons with those quantum numbers in. Principal Quantum Number 2. Signifies the size of the electron cloud.
How many electrons can have the quantum numbers n 5 ℓ 0 ml0. Azimuthal Quantum Number l. Together a specification of all.
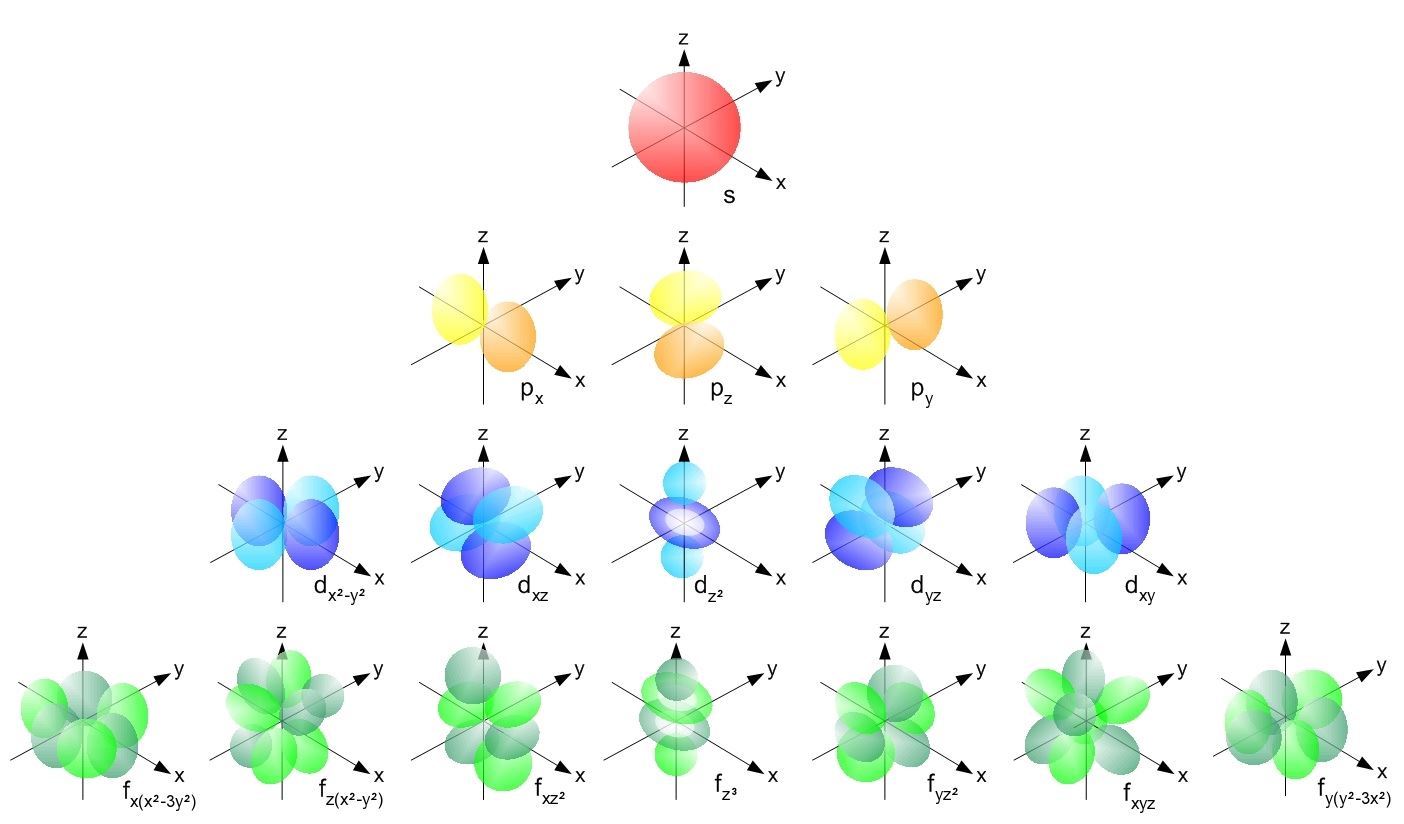
It can have values from l0 to ln-1. M ℓ or m - magnetic quantum number. B A contour representation of a p orbital.
Quantum number and their signifiance. The quantum number that indicates the. To completely describe an electron in an atom four quantum numbers are needed.
N 3 number of electrons. PRINCIPAL QUANTUM NUMBER n - Represents the main energy level or shell occupied by an electron. Angular Momentum Quantum Number o.
The principle quantum number n describes the energy and distance from the nucleus and represents the shell. Consequently do learn the significance of quantum numbers in detail. The first three that came to my mind and the most popular ones are the following.
The azimuthal quantum number l is related to the geometrical shape of the orbital. There are four quantum numbers. The significance of these quantum numbers are - Principal Quantum Number n.
N 4 number of electrons. Learn about atomic orbital the four quantum numbers principal angular momentum magnetic and spin and how to write quantum. Because n describes the most probable distance of the electrons from the nucleus the larger the number n is the farther the electron is from the nucleus the larger the size of the orbital and the larger the atom is.
Theprinciple quantum number is the energy level of an electron. Angular Momentum Quantum Number l- indicates the shape of the orbital. Theangular momentum number is the shape of the orbital holding theelectron.
The first quantum number is the principle quantum number n. A A contour representation of an s orbital. The following four quantum numbers can be used to fully characterise all of the characteristics of an atoms electrons.
The four quantum numbers are the principle quantum number n the angular momentum quantum number l the magnetic quantum number ml and the electron spin quantum number ms. MAGNETIC QUANTUM NUMBER ml - Represents the. Four quantum numbers are used to describe electrons.
Magnetic Quantum Number. The energy of an electron in an atom. This quantum number can be 1 2 3.
100 2 ratings There are four quantum numbers used to describe the distribution of electron density and the values of other conserved quantities in the dynamics of a quantum system eg. 5 rows Quantum Numbers Principal Azimuthal Magnetic and Spin - The set of numbers used to. The original three quantum numbers describe properties of the electron cloud.
Signifies the shape of the electron cloud. In chemistry and quantum physics quantum numbers describe values of conserved quantities in the dynamics of a quantum systemQuantum numbers correspond to eigenvalues of operators that commute with the Hamiltonianquantities that can be known with precision at the same time as the systems energy and their corresponding eigenspaces. Energy n angular momentum ℓ magnetic moment m ℓ and spin m s.
The second quantum number is the orbital quantum number or the angular momentum quantum number l. List the four quantum numbers and describe their significance. Principal Quantum number n Angular Momentum Quantum Number l Magnetic Quantum Number m Spin Quantum Number 12 and -12 The n gives the energy level of the electron then the l which is the sublevel then m which tells you which orbital it is in and last s which tells you if the electron is going to have a.
N - principal quantum number. N 2 number of electrons. The four quantum numbers are the principle quantum number n the angular momentum quantum number l the magnetic quantum number ml and the electron spin quantum number ms.
It is most closely related to the shell or orbits of the Bohr model. The value of l may be zero or a positive integer less than or equal to n1 n is the principal quantum number. The value of n ranges from 1 to the shell containing the outermost electron of that atom.
It is always a. N values are positive integers only. N is the principal quantum number.
All four quantum numbers are required when applying another. The first quantum number describes the electron shell or energy level of an atom. It can have ant positive value 123 and so on.
M s or s - spin quantum number.

Quantum Numbers Chart Physicscatalyst S Blog

Quantum Numbers Explained Chemistry Help Chemistry Lessons High School Chemistry
What Are Quantum Numbers Qs Study
Quantum Number Of Atom To Study The Fine Structure Of An Atom By Chemistry Topics Atomic Theory Medium
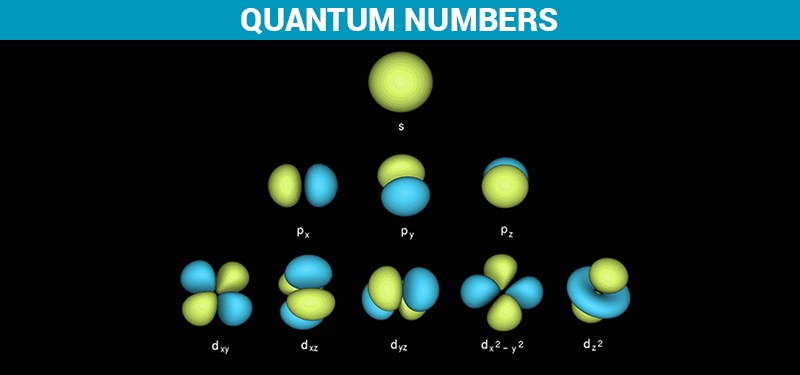
Quantum Numbers Principal Azimuthal Magnetic And Spin Definition Detailed Explanation Videos And Faqs Of Quantum Numbers

Question Video Determining The Quantum Numbers That Represent An Electron In An Atom Nagwa
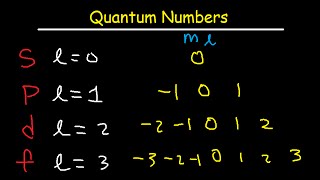
How To Determine The 4 Quantum Numbers From An Element Or A Valence Electron Youtube
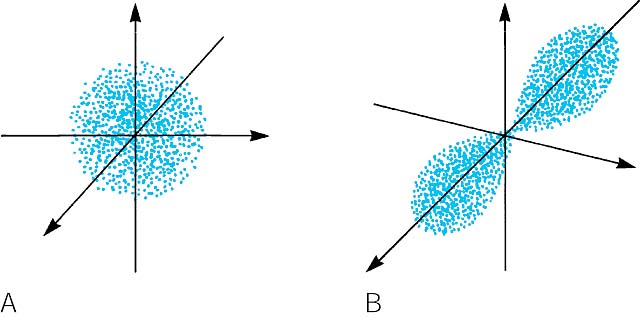
The Physical Significance Of The 4 Quantum Numbers
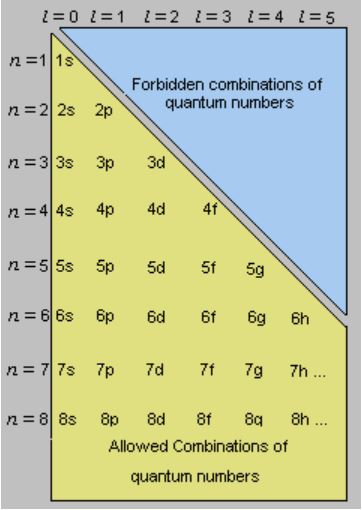
Quantum Numbers Principal Azimuthal Magnetic And Spin Definition Detailed Explanation Videos And Faqs Of Quantum Numbers

Quantum Numbers The Easy Way Youtube
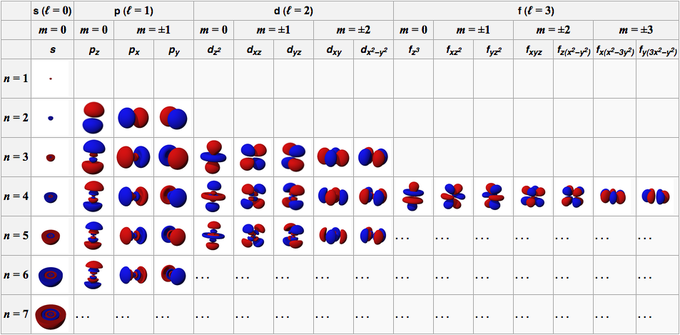
Quantum Numbers Introduction To Chemistry

Quantum Numbers Chemistry For Non Majors

2 2 Atomic Orbitals And Quantum Numbers Chemistry Libretexts

Quantum Numbers Definition Examples And Chart

Quantum Number Orbital Definition Formula Diagram Shape

Quantum Numbers Principal Azimuthal Magnetic And Spin Definition Detailed Explanation Videos And Faqs Of Quantum Numbers
Comments
Post a Comment